Aims and Scope
 Journal of Pharmaceutical and Biopharmaceutical Research (JPBR) (eISSN:2630-533X) is an open access, continuously published, international, refereed journal. The aim of the journal is to provide the authors a timely and peer reviewed process for evaluation and publication of their manuscripts. All articles submitted to JPBR will undergo a rigorous double-blind peer review, and all published articles can be downloaded and read for free. JPBR will pay wide attention to the trends in related fields and insist on publishing original research work of highest quality.
Journal of Pharmaceutical and Biopharmaceutical Research (JPBR) (eISSN:2630-533X) is an open access, continuously published, international, refereed journal. The aim of the journal is to provide the authors a timely and peer reviewed process for evaluation and publication of their manuscripts. All articles submitted to JPBR will undergo a rigorous double-blind peer review, and all published articles can be downloaded and read for free. JPBR will pay wide attention to the trends in related fields and insist on publishing original research work of highest quality.
JPBR publishes high quality original research work, reviews, and short communications in the following areas:
Pharmaceutical Sciences:
• Pharmaceutics
• Pharmacology and Toxicology
• Medicinal Chemistry
• Physical Pharmacy
• Pharmaceutical Analysis
• Chromatography and Hyphenated Techniques
• Pharmacognosy and Phytochemistry
• Nanotechnology for Pharmaceutical Drug Formulations
Biopharmaceutical Sciences:
• Biochemistry
• Biotechnology
• Molecular Biology
• Immunology and Microbiology
 |
ISSN: 2630-533X
Abbreviation: J Pharm Biopharm Res
Editor-in-Chief: Prof. Pal Perjesi (Hungary)
Publishing Frequency: Continuous publication
Article Processing Charges (APC): Click here for more details
Publishing Model: Open Access |



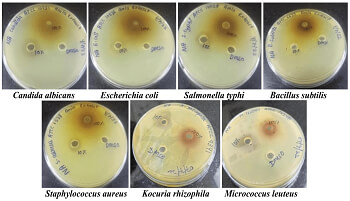
 Ritu Tiwari, Gaurav Sanjay Mahalpure, Sakshi Mahalpure, Anuanshika Tiwari
Ritu Tiwari, Gaurav Sanjay Mahalpure, Sakshi Mahalpure, Anuanshika Tiwari




